NNNS: Approval by the FDA (2011)
Click here to return to the Nutritive and non-nutritive sweetener evidence analysis project.
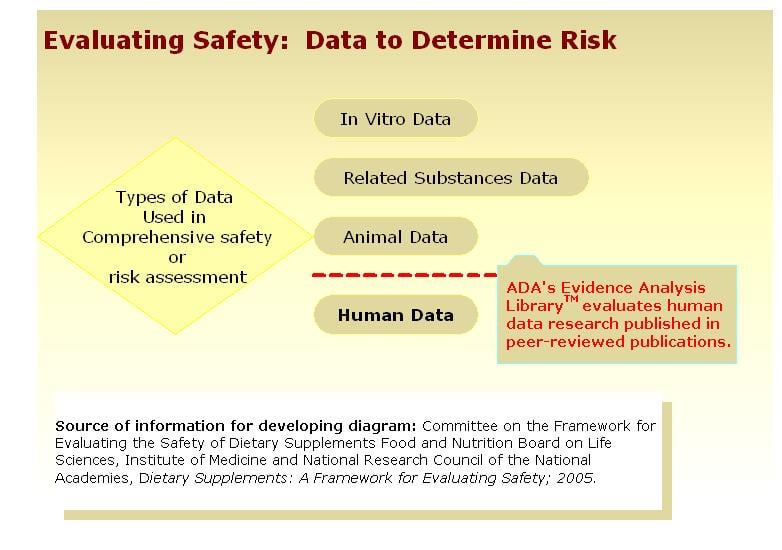
Non-nutritive sweeteners and FDA
Only non-nutritive sweeteners approved by the FDA and considered to be safe for human consumption are included in the N&NNS evidence analysis project. These include: acesulfame-K, aspartame, neotame, saccharin, stevia, and sucrolose.
Background: From time to time, questions arise about the safety of non-nutritive sweeteners particularly in regard to cancer, seizures, pregnancy and other toxicities. The FDA has approved the use of non-nutritive sweeteners and they are considered to be safe. These FDA data are part of the pre-market assessment, are not published in peer reviewed journals and are not part of the ADA evidence analyzed for this EAL project. However, ADA has provided you with a link to the U.S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition website.
External Links:
- FDA Click here to go to the "Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients" from the U.S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition website.
- NCI Click here to go to the "National Cancer Institute Fact Sheet" regarding artificial sweeteners and cancer on the NCI website